Others
DA-1241

- DA-1241 is a once-daily, orally available, chemical drug candidate currently in early clinical development as a potential treatment for NASH and type 2 diabetes.
- All rights for development and marketing of DA-1241 outside of Korea have been licensed out to NeuroBo Pharm (NASDAQ: NRBO).
Clinical unmet needs
- NASH is a silently progressing metabolic liver disease in which the liver has more than 5% fat and has varying degrees of inflammation and fibrosis.
- Cardiovascular disease (CVD) is a leading cause of morbidity and mortality in the NASH patients1. As metabolic diseases characterized by calorie excess, particularly diabetes and obesity, become more common worldwide, the incidence of new NASH is increasing. Long-term persistence of hyperglycemia and/or hyperlipidemia increases the incidence of cardiovascular disease2.
- Therefore, improving liver lesions and efficiently controlling comorbidities in NASH patients are important for effectively reducing the risk of cardiovascular events. Up to now, there are no anti-NASH drugs approved by the FDA.
MoA
(Mechanism of Action)
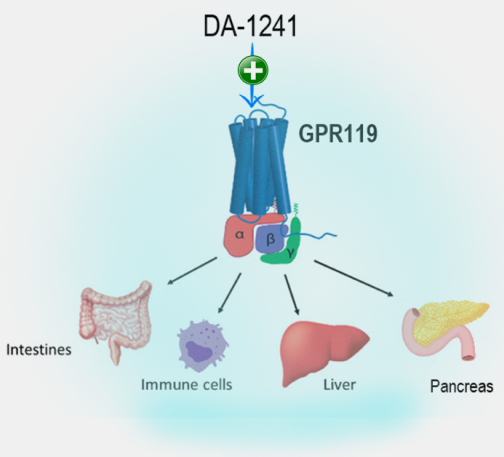
- DA-1241 is a new chemical agent activating G protein-coupled receptor 119 (GPR119) mainly in the pancreas, intestine, and liver.
- In non-clinical studies, GPR119 activation by DA-1241 in hepatocytes, macrophages, and hepatic stellate cells inhibits lipid accumulation, immune cell infiltration, and the production of collagen fibers in the liver, directly ameliorating NASH pathophysiology such as steatosis, inflammation, and fibrosis.
- Moreover, GPR119 has a distinctive role in glucose and lipid metabolism via stimulating secretion of insulin and glucagon-like peptide-1 (GLP-1) in pancreatic beta cells and intestinal L-cells, respectively. Therefore, DA-1241 can also provide additional metabolic benefits in NASH patients with common comorbid metabolic diseases such as type 2 diabetes and dyslipidemia.
- In Phase 1b clinical trials, the safety and efficacy of DA-1241 monotherapy were successfully confirmed. DA-1241 was well-tolerated in U.S. type 2 diabetic patients. GPR119 activation by DA-1241 treatment increased the plasma levels of gut peptide hormones (total GLP-1, PYY, GIP) as plasma concentrations of DA-1241 went up and showed a persistent and similar postprandial glucose-lowering activity to JANUVIA® (an oral anti-diabetic agent as a dipeptidyl peptidase inhibitor) in a head-to-head trial after 8 weeks of once daily oral treatment.
Publications
-
A novel GPR119 agonist DA-1241 preserves pancreatic function via the suppression of ER stress and increased PDX1 expression. Kim MK, Cheong YH, Lee SH, Kim TH, Jung IH, Chae Y, Lee JH, Yang EK, Park H, Yang JS, Hong KW. Biomed Pharmacother. 2021 Dec;144:112324. doi: 10.1016/j.biopha.2021.112324. PMID: 34678732
-
DA-1241, a Novel GPR119 Agonist, Improves Hyperglycaemia by Inhibiting Hepatic Gluconeogenesis and Enhancing Insulin Secretion in Diabetic Mice. Kim Y, Lee SW, Wang H, Kim RH, Park HK, Lee H, Kang ES. Diabetes Metab J. 2022 Mar;46(2):337-348. doi: 10.4093/dmj.2021.0056. PMID: 35052026
-
GPR119 activation by DA-1241 alleviates hepatic and systemic inflammation in MASH mice through inhibition of NFκB signaling. Lee SH, Park H, Yang EK, Lee BR, Jung IH, Kim TH, Goo MJ, Chae Y, Kim MK. Biomed Pharmacother. 2023 Aug 30;166:115345. doi: 10.1016/j.biopha.2023.115345. PMID: 37657264

