Others
DA-8010 (velufenacin)

Unmet needs
- OAB (overactive bladder) is defined by the International Continence Society as urinary urgency, with or without urge incontinence, usually with urinary frequency and nocturia. The estimated overall prevalence is 11.8 - 22% of adults across Europe and the United States. The prevalence increases with age, affecting approximately 30% of individuals over 65 years of age. Symptoms of OAB have a significant negative impact on health-related quality of life (HRQoL) that is similar to that observed with diabetes mellitus. It is associated with an increased risk of sleep disturbance, anxiety, low self-esteem, urinary tract infection and injury in a fall. OAB imposes a substantial economic burden. Thus, effective treatment of OAB is essential to improve patients’ HRQoL and to decrease the burden of disease.
- Muscarinic receptor antagonists are currently the first-line pharmacotherapy for OAB. A meta-analysis of 83 clinical trials supports the view that antimuscarinics are efficacious in reducing the frequency of incontinence, micturition and urgency. However, the currently available antimuscarinics are associated with a relatively high incidence of side effects, such as dry mouth, constipation, headache and blurred vision, which often leads to discontinuation of treatment. In addition, the rate of patients persisting with the current antimuscarinics is low due to unmet treatment expectations and side effects. A high proportion discontinue treatment, with less than 25% remaining on therapy at 1 year. Therefore, there is a need for new OAB treatments with high efficacy and fewer side effects.
MoA
(Mechanism of Action)
- DA-8010 is a novel, potent, and selective Muscarinic receptor 3 antagonist being developed by Dong-A ST for the treatment of OAB.
- Muscarinic receptor antagonists act during the storage phase, increase bladder capacity, decrease involuntary contraction and delay the initial urge to void. Urinary bladder smooth muscle is predominantly innervated by parasympathetic cholinergic nerves, with acetylcholine being the main transmitter. Muscarinic receptors mediate urinary bladder contraction during the voiding phase, and control detrusor tone during the filling phase. The urinary bladder smooth muscle contains a mixed population of muscarinic M2 and M3 receptors. Although fewer in number than M2 receptors (1:3 ratio), M3 receptors are primarily responsible for the control of bladder contraction.
Competitiveness
- In nonclinical studies, compared to other antimuscarinics in clinical use, DA-8010 was shown to have the highest binding affinity and the most potent antagonism for the human M3 receptor, with significant selectivity and potency for M3 receptors versus M1 and M2 receptors. DA-8010 had greater selectivity for bladder smooth muscle than salivary gland cells and tissues, suggesting it has a decreased potential for causing dry mouth symptoms, and was quicker acting and longer acting in bladder. Furthermore, DA-8010 caused little or no constipation at a dose having an effect on the bladder. DA 8010 was also highly selective for the bladder over the heart, brain and eye, indicating a decreased potential for cardiac toxicity, central nervous system and ocular adverse effects. The urodynamic data from cyclophosphamide-induced OAB rat model and partial bladder outlet obstructed rat model revealed that DA-8010 increased micturition interval without debilitating bladder function. In addition, DA-8010 improved the biochemical and histological findings of detrusor overactivity induced by partial bladder outlet obstruction in a rat model.
- A phase 1 clinical study was conducted to evaluate the safety, tolerability, and pharmacokinetic characteristics of single and multiple oral doses of DA-8010 in healthy adult subjects in the UK. A single dose of DA-8010 and multiple doses of DA-8010 were well-tolerated and there was no food effect in relation to the tolerability profile of DA-8010.
- A phase 2 clinical study was conducted to evaluate the efficacy and safety of DA-8010 in patients with overactive bladder in Korea. Both DA-8010 2.5 mg and 5 mg showed therapeutic efficacy for OAB without serious ADRs. Therefore, both dosages of DA-8010 can advance to a subsequent large-scale phase 3 trial (On-going phase 3 trial in Korea).
- Overall, these findings suggest that DA-8010 with its greater efficacy for overactive bladder and potentially less side effects than currently available drug therapies, offers the potential for an improvement in OAB patients’ treatment compliance and quality of life.
- Potential of a Best-in-Class OAB Medication
- Attrative Preclinical and Clinical profiles
- Improvement of OAB patients' Compliance and Quality of Life

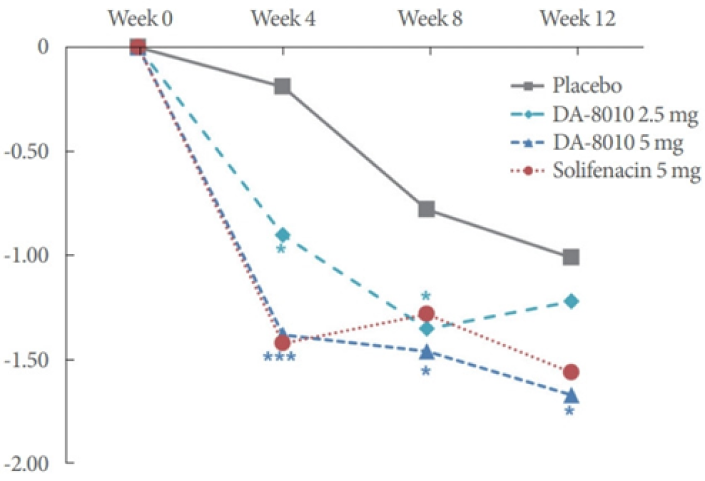
- Mean changes of 24-hour frequency (primary endpoint) in OAB patients (phase 2 clinical trial) *P<0.05. ***P<0.001.
Publications
- European Journal of Pharmacology, 843, 240, 2019
- BMC Urology 20, 41, 2020
- Int Neurourol J. 26, 119, 2022
- Pharmacology Research & Perspectives 11, e01040, 2023
- American Urological Association, 2016, 2017, 2019, 2021

